|
| Main Menu - click above |
Michael Faraday breathed in mercury vapour virtually every day for over 25 years. His mental health suffered badly from this exposure to quicksilver fumes.
An early scientific study of mercury poisoning was in 1923–6 by the German inorganic chemist, Alfred Stock, who himself became poisoned, together with his colleagues, by breathing mercury vapour that was being released by his laboratory equipment—diffusion pumps, float valves, and manometers—all of which contained mercury, and also from mercury that had been accidentally spilt and remained in cracks in the linoleum floor covering. He published a number of papers on mercury poisoning, founded a committee in Berlin to study cases of possible mercury poisoning, and introduced the term micromercurialism. from Wikipedia
Stock suggested in his 1926 Zeitschrift für angewandte Chemie article, "The Danger of Quicksilver Vapour," that Michael Faraday suffered from micromercurialism.
Certainly Faraday suffered greatly from ill-health, as illustrated by these excerpts from "The Life and Letters of Faraday" by Bence Jones 1870 -
1841
1844
1847
1847
1858
1861
Faraday's work brought him in constant proximity to mercury. He outlines the liquid metal's uses in his book "Chemical Manipulation" excerpts below. Full book here, excellent article here
Mercurial trough used to collect gases that are soluble in water
"Newman also has a much smaller trough for the use, though in a confined manner, of jars 1.5 inches in diameter and six inches in length; it has only 30 square inches of shelf room. It requires 20 lbs.of mercury to fill it ... A mercurial trough should always stand in a tray, and likewise have a cover to keep out dust and dirt when not in use. Its place should be upon the table grooved round the edge, that waste mercury may be avoided as much as possible. When the metal is spilled it is best collected by being swept together and then gathered up by a card."
To fill a capped jar with liquid mercury
"When a capped jar is to be filled with mercury, by the assistance of the mouth, the jar should be inclined as much as possible to diminish the height of the column of air within it, as well as the labor attending the operation; then by applying the mouth to the stopcock and using it to exhaust in a manner almost the reverse of that described for blow pipe practice, the air may be withdrawn and the mercury gradually raised until it fills the jar."
High temperature baths
"To achieve bath temperatures above 212°F one can use liquid mercury as the fluid. If the experiments be made altogether in tubes, a temperature of 600°F may easily be communicated by means of it; but if the bath be an open vessel, a dish or a crucible for instance, then temperatures higher than 450°F should not be given to it; for the metal soon rises in vapor"
Electrostatic generators
"It is often advantageous, especially when the machine is required in haste, to hold a piece of silk with some amalgam upon it against the plate or cylinder, whilst it is turned, and also to rub up the surface of the amalgam upon the rubber with the same amalgamated silk."
Excerpt below from Faraday's Health Problems, by James F. O'Brien, published in The Bulletin for the History of Chemistry, 1991. pdf here
 |
| Please help beat cancer - DONATE click above |
 |
A STEAMPUNK NOVEL, FULL OF
ANARCHIC EXPERIMENTAL SCIENCE
"Hodges emitted a scream the like of which
I hadn't heard since his scrotum was burned off
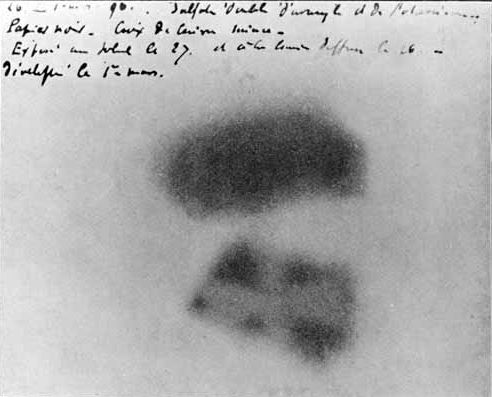
Unrelated to this post, below is an example of
eclectic science esoterica
Photographic plate made by Henri Becquerel showing effects of exposure to radioactivity.
Opisthoproctus soleatus, a species of barreleye (Opisthoproctidae)
Illustration by Brauer, A., 1906. A live barreleye below.
 |
| Main Menu - click above |